A Next-Generation IVL Tool for Calcification and PAD Management
Calcification in peripheral arteries is not uncommon and presents significant challenges to endovascular interventions, mainly due to reduced vascular compliance in these stenotic lesions.1 Balloon angioplasty is frequently associated with residual stenosis and dissection, and it often requires stenting.2 Stents in these densely calcified lesions tend to remain compressed, resulting in acute loss of patency and other long-term complications such as stent fractures and early restenosis. Intraluminal plaque-modifying techniques such as atherectomy do not address vessel compliance and medial calcification. Additionally, atherectomy in calcified lesions has significantly higher rates of procedural embolization (13%), despite the use of distal filters, dissections (14%), and perforations (3%).3
The Shockwave Intravascular Lithotripsy (IVL) System (Shockwave Medical) is a novel balloon catheter–based device that uses sonic pressure waves to disrupt calcified lesions modifying vessel compliance. This allows for safe and effective luminal gain with minimal need for stenting.4 Numerous clinical trials—including DISRUPT PAD and DISRUPT BTK—have demonstrated the safety and efficacy of the IVL balloon.4-6 In my practice, IVL has become our new gold standard in the management of calcified arterial lesions. Some of the challenges with the previous generation of IVL catheters include shorter balloon length and larger crossing profile. With the introduction of Shockwave E8, a new generation of catheters is now available in a longer balloon length (8 cm), in a longer working length (150 cm), and with more pulses (400), allowing for the treatment of 30cm+ lesions (Figure 1). The following case report describes my initial experience with these new and improved Shockwave E8 IVL balloons.
Figure 1. Shockwave E8 balloon.
Case Study: Long-Segment Tibial Disease in Patient with Non-Healing Wound
CASE PRESENTATION
A man in his mid 90’s with a recent COVID-19 infection and known peripheral artery disease presented with a non-healing ulcer located on the medial aspect of his foot at the head of the first metatarsal bone. Plain x-ray of the foot revealed cortical destruction involving the distal aspect of the right first metatarsal bone adjacent to a soft tissue defect, which was concerning for osteomyelitis. Arterial duplex ultrasound suggested significant tibial disease. Diagnostic angiography of the right leg revealed distal occlusion of the posterior tibial (PT) artery, and the peroneal artery did not reconstitute the PT artery in the foot. The anterior tibial (AT) artery had multiple areas of 99% stenosis and was the dominant runoff to the foot (Figure 2).
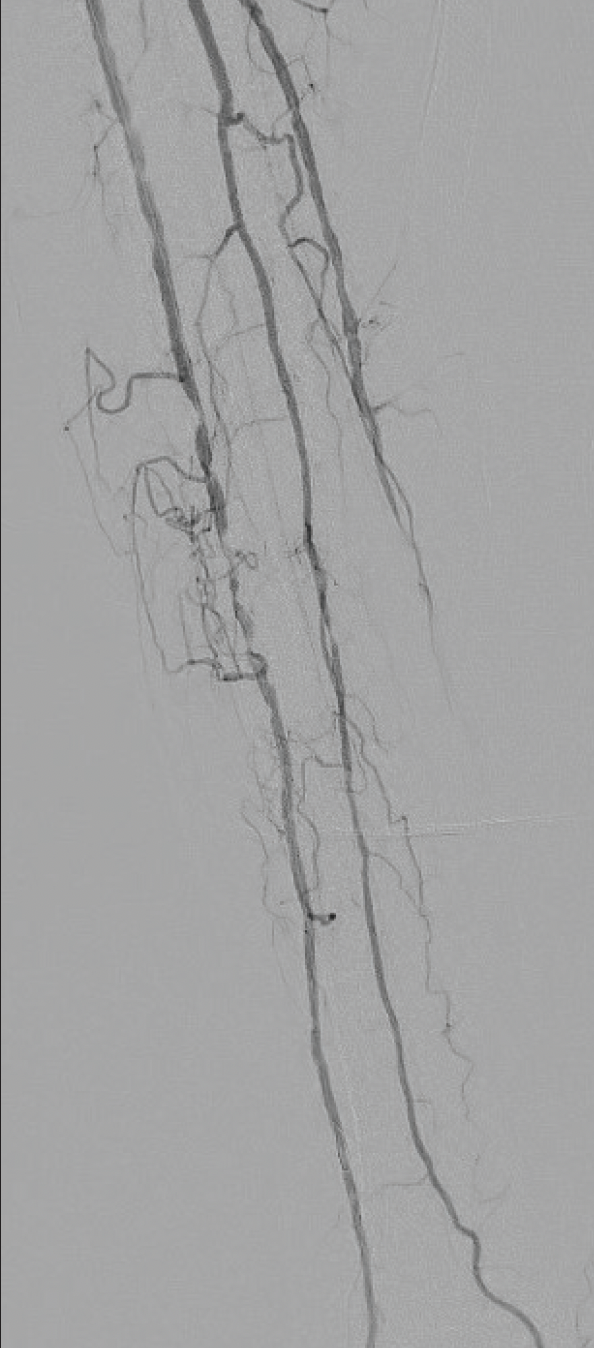
Figure 2. Diagnostic angiogram demonstrated high-grade lesions in the AT artery.
PROCEDURAL OVERVIEW
The AT artery lesions were crossed with a 0.014-inch wire, and intravascular ultrasound (IVUS) images were obtained. IVUS demonstrated dense circumferential calcification. The lesion was treated with a 3.0mm Shockwave E8 balloon. The initial waist in the balloon was noted at 2 atm (Figure 3) and resolved within two cycles. Repeat angiography demonstrated no residual stenosis (Figure 4). The patient developed a palpable dorsalis pedis pulse and subsequently underwent a first-toe ray amputation with adequate healing. The amputation site healed with no issues.
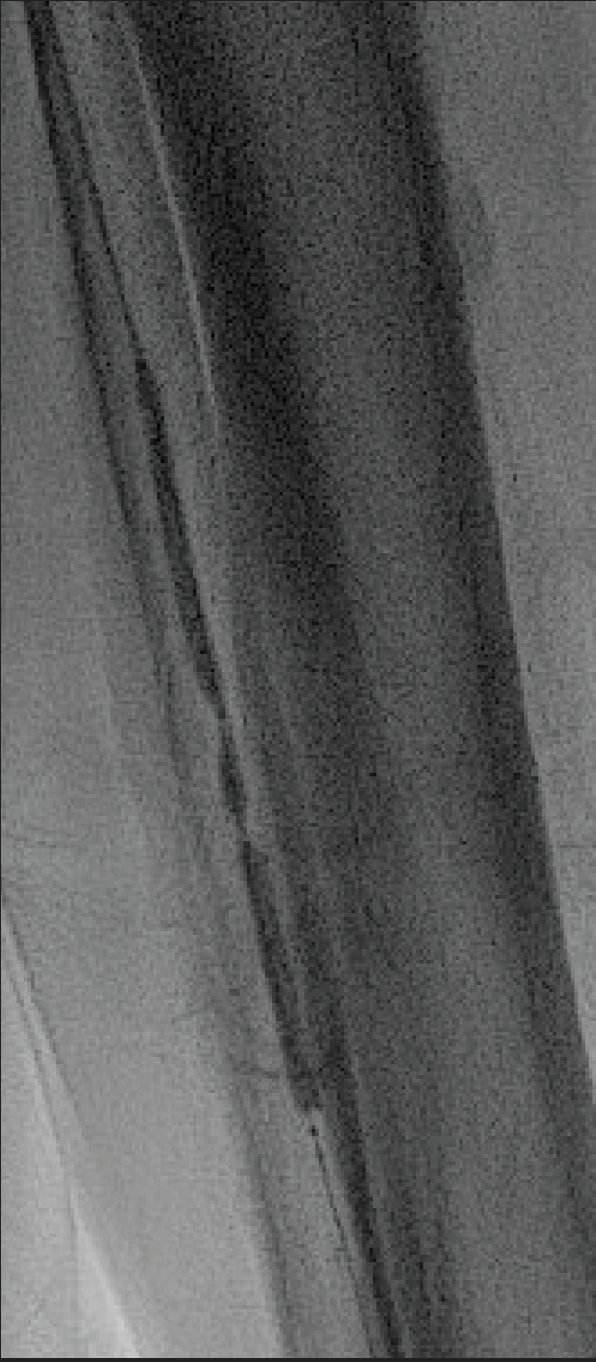
Figure 3. Low-pressure (2 atm) angioplasty with a Shockwave E8 balloon.
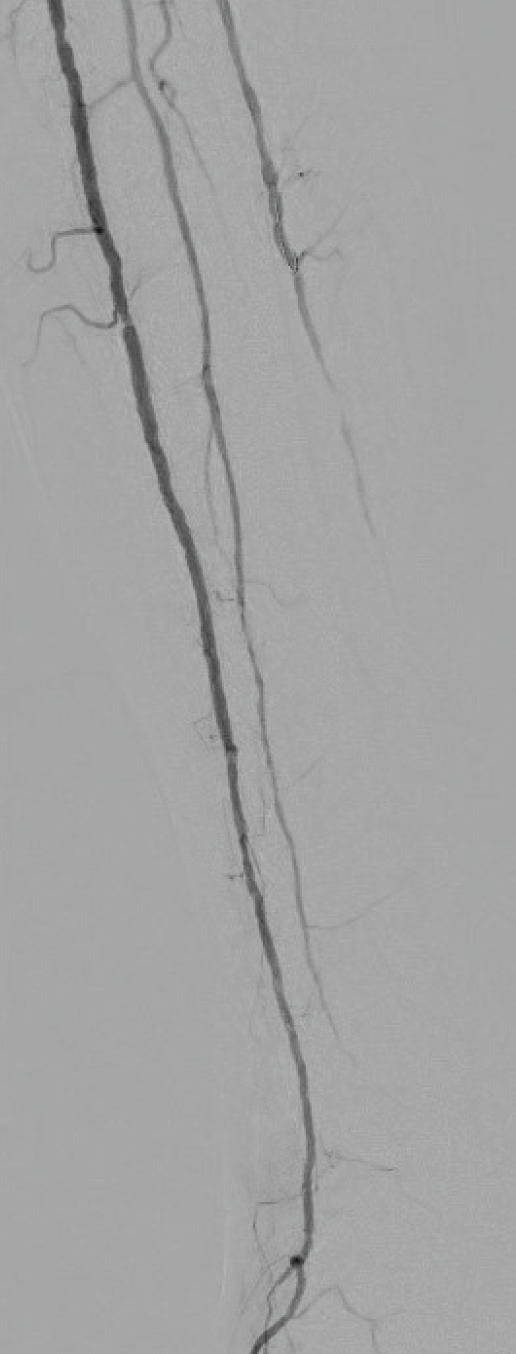
Figure 4. Completion angiogram demonstrated inflow flow via the AT artery with no residual stenosis.
DISCUSSION
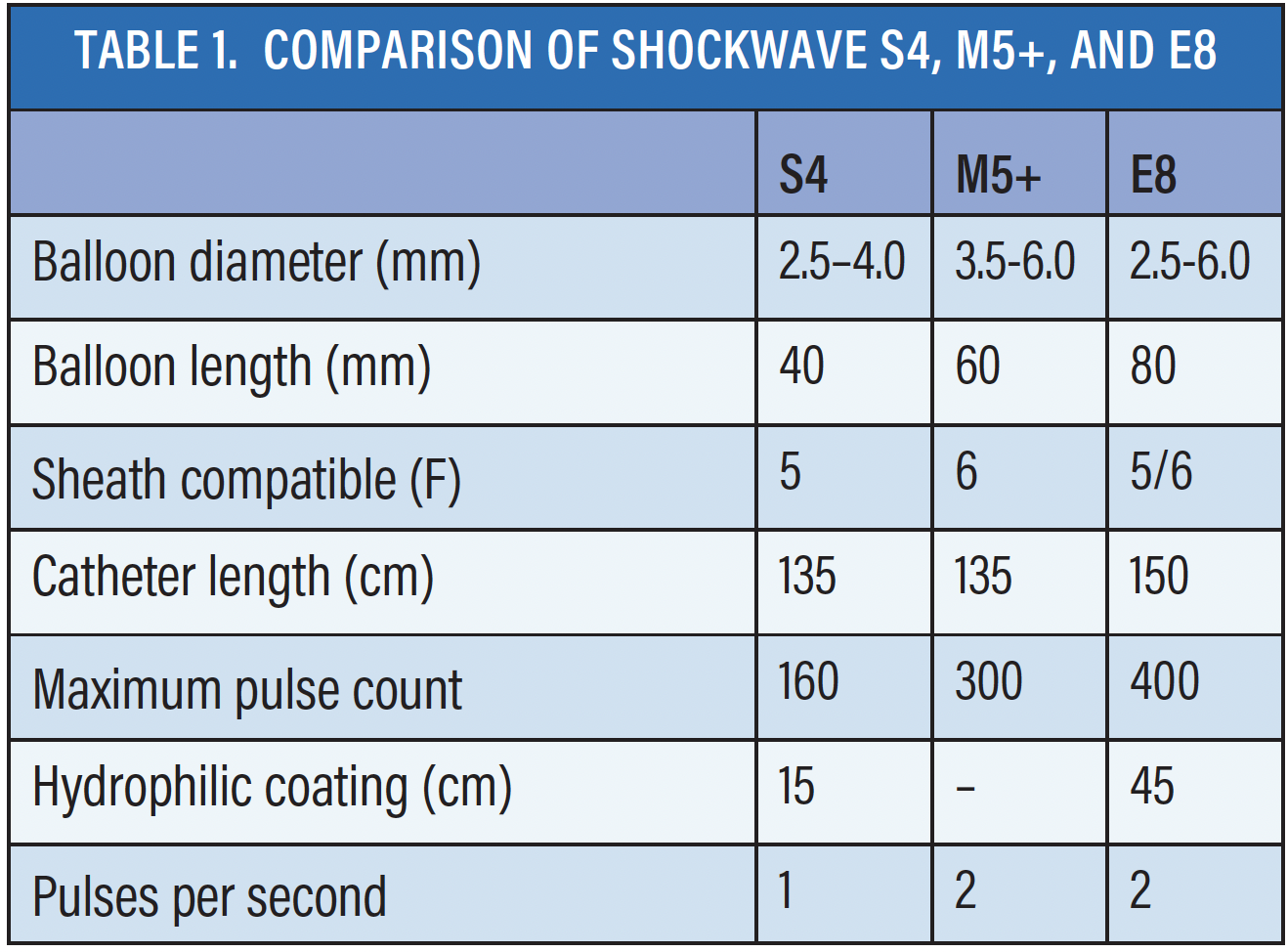
IVL presents a quantum leap in the management of vascular calcification. The new Shockwave E8 catheters offer a variety of enhancements that simplify the endovascular procedure, thereby making it more user-friendly (Table 1). The longer balloon length (8 cm) and increased number of pulses (400 pulses at 2Hz) provide the ability to treat long lesions, therefore allowing for fewer balloon exchanges and for management of highly resistant vascular calcification (Figure 1). Longer hydrophilic coating (45 cm) and a more tapered entry profile allow for enhanced deliverability.* With the longer usable catheter length (150 cm), I have been able to treat some tibial lesions from a radial approach. Additionally, the energy profile is consistent across the entire length of the Shockwave E8 balloon, providing uniform treatment across the length of the balloon. Predilation or other adjunctive techniques were not required in our initial 12-patient experience, where eight of 12 had chronic total occlusions and three patients underwent radial access.
The new Shockwave E8 IVL balloons allow physicians to address complex lesions with efficient treatment time.

*83% of 183 physicians in the Limited Market Release rated Shockwave E8 to be more deliverable than the previous generation catheters
SPL-73283 Rev. A
Dr. Kasirajan is a paid consultant of Shockwave Medical.
In the United States: Rx only.
Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary, carotid or cerebral vasculature.
Contraindications— Do not use if unable to pass 0.014’’ (M5, M5+, S4, E8) or 0.018‘’ (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings— Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator’s Manual.
Precautions— Use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects— Possible adverse effects consistent with standard angioplasty include–Access site complications–Allergy to contrast or blood thinner–Arterial bypass surgery—Bleeding complications—Death—Fracture of guidewire or device—Hypertension/Hypotension—Infection/sepsis—Placement of a stent—renal failure—Shock/pulmonary edema—target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)—Device malfunction or failure—Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events.
The Shockwave E8 Peripheral Catheter is not available in all geographies. Please contact your local Shockwave representative for specific country availability.
www.shockwavemedical.com/ifu